Abstract
Pneumonia is a significant global health
concern, particularly among children under five and the
elderly. Early detection is crucial for effective treatment
and can save lives. Chest X-rays (CXRs) are the gold
standard for diagnosing pneumonia, but manual
interpretation is time-consuming and inconsistent,
especially in areas with a shortage of medical
professionals. This study explores the application of
YOLO (You Only Look Once), a fast and accurate image
analysis tool, to detect pneumonia in CXRs. We utilized
the “Chest X-Ray Images (Pneumonia)” dataset from
Kaggle and evaluated various YOLO models for speed
and accuracy in practical healthcare settings. By
incorporating attention mechanisms and making model
adjustments, we found that YOLO achieved over 91%
accuracy, significantly reducing the time required for
radiologists to assess patients. Our findings demonstrate
that AI can be effectively implemented in healthcare,
bridging the gap between technology and real-world
applications, making pneumonia diagnosis rapid and
accessible.
Methodology
The dataset utilized in this study is sourced from
Kaggle, consisting of 5,856 chest X-ray images classified
into two categories: normal and pneumonia (both viral and
bacterial). The dataset is organized into three main folders:
training, validation, and testing, with each folder containing
subfolders for normal and pneumonia cases. Following a
70-15-15 split ratio, approximately 4,099 images are
allocated for training, while the validation and testing sets
each contain around 879 and 878 images, respectively. This
dataset structure enables comprehensive model evaluation.
In the preprocessing stage, chest X-ray images are
resized to 640x640 pixels to match YOLO11's standard
input requirements. Additionally, pixel values are
normalized to a range of 0-1 to optimize the model's
learning process. To increase data variability and prevent
overfitting, data augmentation techniques such as rotation,
flipping, brightness adjustment, and random cropping are
applied.
-
Model Architecture
The YOLO11 architecture used in this research is
modified for binary classification tasks. The model employs
CSPDarknet as the backbone and PANet as the neck, while
the detection head is specifically modified for pneumonia
classification. This architectural adjustment allows the
model to focus on specific classification tasks while
maintaining the robust feature extraction capabilities
inherent in the YOLO11 architecture. -
System Implementation
The implementation of the pneumonia detection system
involves developing a backend using the Flask framework
and a simple web-based frontend. The backend is
responsible for processing uploaded images, making
predictions using the trained model, and returning
prediction results. The frontend provides a user-friendly
interface that allows users to upload X-ray images and
easily view prediction results. -
Model Evaluation
Model evaluation is conducted using various metrics,
including accuracy, precision, recall, F1-score, and ROC
AUC. The evaluation results demonstrate that the model
can detect pneumonia with high accuracy, making it a potential tool to assist in early pneumonia diagnosis in
clinical practice. -
System Architecture Overview
Figure 1 illustrates the general architecture of our
pneumonia detection system, which consists of several
interconnected stages. The process begins with data
collection from the Kaggle dataset, followed by
comprehensive preprocessing steps, including image
resizing, noise reduction, image enhancement, data
augmentation, and class labeling to categorize images as
either Normal or Pneumonia. The preprocessed data is then
split into three distinct sets: 70% for training, and 15% each
for validation and testing purposes.
During the model training phase, feature extraction, loss
calculation, and model optimization are performed to
ensure optimal performance. The validation process
continuously monitors various metrics, including accuracy
and loss, while generating confusion matrices to assess
model reliability. The testing phase evaluates the model's
effectiveness through precision, recall, and F1-score
measurements. Finally, the trained model is deployed
through web integration, making it accessible for practical
applications.
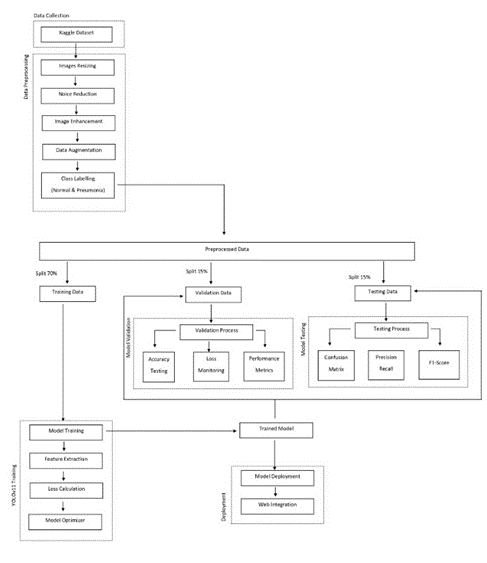
This integrated architecture demonstrates a well-structured approach to developing an automated pneumonia detection system, combining comprehensive data preprocessing, robust model training, thorough evaluation processes, and practical deployment considerations. The inclusion of multiple validation and testing metrics ensures the system's reliability and effectiveness in real-world applications.
Results
The results obtained from the pneumonia detection system using the YOLO11 architecture demonstrate promising efficacy in classifying chest X-ray images. This chapter presents the evaluation metrics, confusion matrices, and practical implementation details, followed by a discussion of the implications of these findings.
- Evaluation Metrics
The performance of the model was evaluated using accuracy, precision, recall, and F1-score across training, validation, and testing datasets. The results are summarized in the table 1:
| Metrics | Training | Validation | Testing |
|---|---|---|---|
| Accuracy | 90.68% | 91.80% | 91.24% |
| Precision | 91.23% | 93.21% | 90.56% |
| Recall | 90.07% | 90.25% | 90.78% |
| F1-score | 90.65% | 91.71% | 90.67% |
These metrics indicate that the model is capable of accurately identifying pneumonia cases while maintaining a low rate of false positives and false negatives.
- Confusion Matrices
Figure 2 illustrates the confusion matrix for the training set, showing that out of 4,099 samples, the model correctly identified 1,866 pneumonia cases and 1,851 normal cases, with 204 false positives and 178 false negatives.
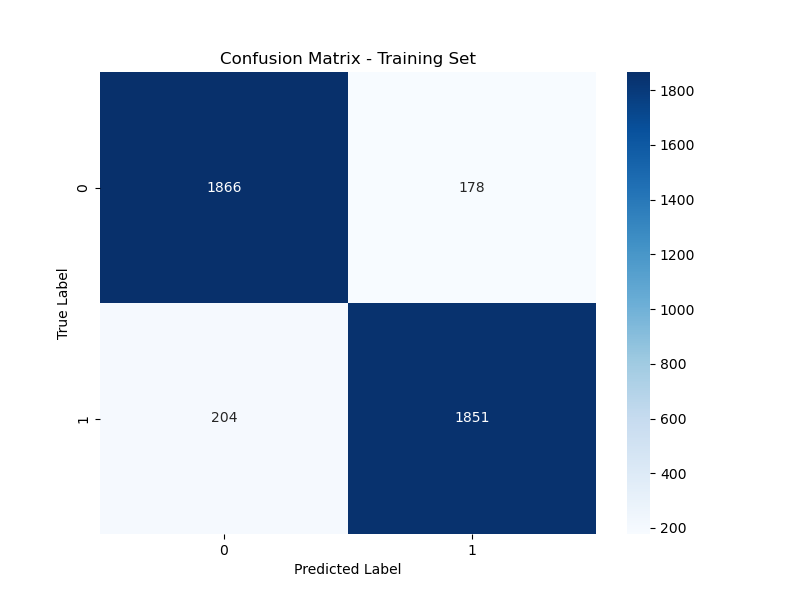
Figure 3 depicts the confusion matrix for the validation set, where the model achieved an accuracy of 91.80%. It correctly predicted 408 pneumonia cases and 398 normal cases, indicating strong generalization capabilities.

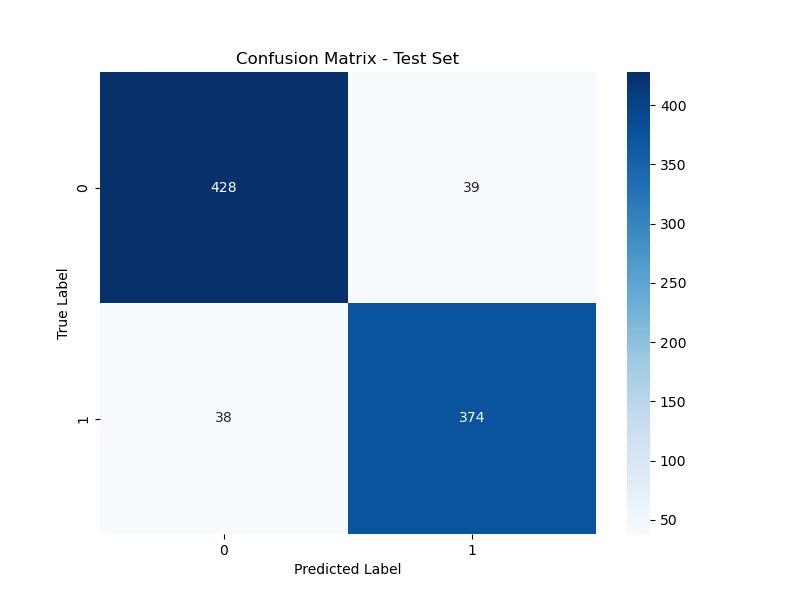
Figure 4 presents the confusion matrix for the test set, confirming the model's consistent performance with an accuracy of 91.24%. The model accurately predicted 428 pneumonia cases and 374 normal cases.
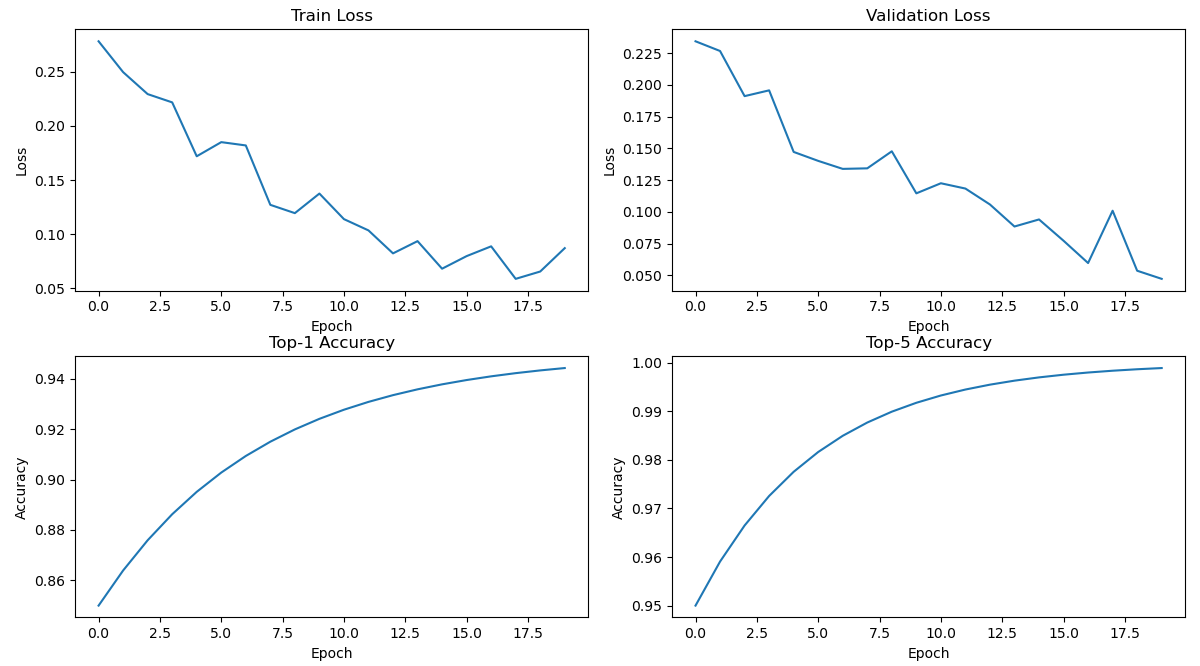
- Training and Validation Loss
The training and validation loss curves are shown in Figure 5. The loss values steadily decreased over the epochs, indicating effective parameter optimization and model learning without significant overfitting.
- Practical Implementation
The practical implementation of the pneumonia detection system was demonstrated through a web-based application. Figure 6 shows the interface where users can upload chest X-ray images for analysis. The system provides instant predictions, enhancing accessibility for medical practitioners.
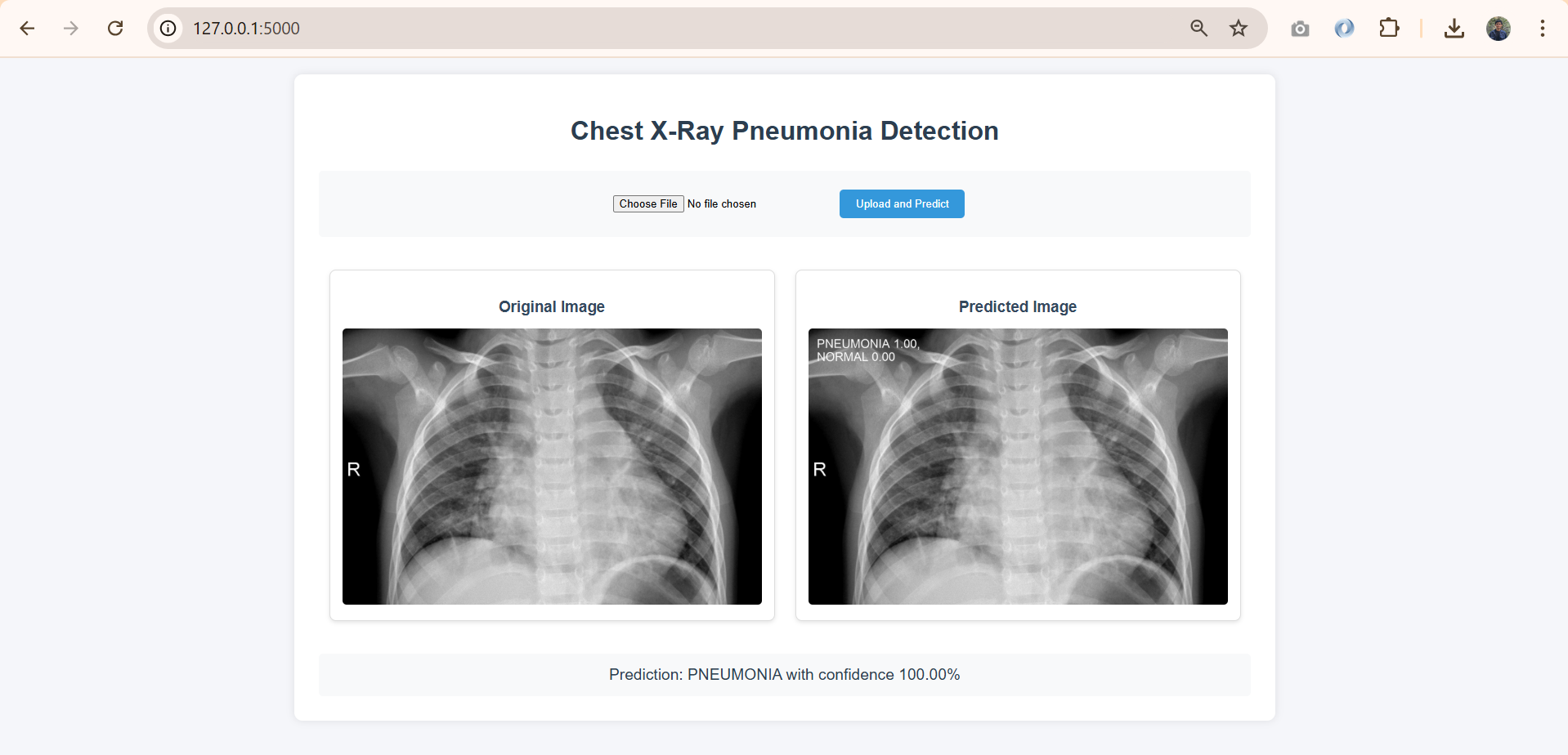
Figure 7 illustrates the results of a prediction, where the model successfully identified pneumonia with 100% confidence. This feature is crucial for clinicians to interpret results effectively.
- Discussion
The results indicate that the YOLO11-based pneumonia detection system is effective and reliable. The high accuracy rates across all datasets suggest that the model can be a valuable tool for assisting in the early diagnosis of pneumonia, which is critical for timely treatment.
The confusion matrices reveal that the model maintains a good balance between sensitivity (recall) and specificity (precision). This balance is crucial in a medical context, where false negatives can lead to undiagnosed pneumonia cases, while false positives can result in unnecessary anxiety and treatment for patients.
The model's ability to generalize well on the validation and test datasets, as evidenced by the high precision and recall rates, further supports its applicability in real-world scenarios. The implementation of a user-friendly web interface allows for easy integration into clinical workflows, making it accessible for healthcare professionals.
In conclusion, this research demonstrates the successful development of an automated pneumonia detection system that not only achieves high statistical accuracy but is also practical for clinical use. Future work may focus on optimizing model performance further, exploring additional data sources, and enhancing the web interface to support a broader range of diagnostic features. The integration of such systems into healthcare settings could significantly improve the speed and accuracy of pneumonia diagnoses, ultimately benefiting patient outcomes.
Conclusion
The research presents a novel pneumonia detection system utilizing the YOLO11 architecture for automated analysis of chest X-ray images. The dataset consists of 5,856 images, divided into training, validation, and testing sets with a 70-15-15 split ratio to enhance model robustness. Key preprocessing techniques included resizing images and normalizing pixel values, along with data augmentation methods such as rotation and brightness adjustments to prevent overfitting.
The model was evaluated using various metrics: it achieved a training accuracy of 90.68%, validation accuracy of 91.80%, and testing accuracy of 91.24%. Confusion matrices illustrated the model's effectiveness in distinguishing between normal and pneumonia-affected images across all datasets. Additionally, a user-friendly web interface was developed, allowing healthcare practitioners to upload images and receive real-time predictions with confidence levels.
The findings highlight the effectiveness of deep learning in medical imaging for pneumonia diagnosis, showcasing the potential of the YOLO11-based system as a valuable diagnostic tool in clinical settings. Continued optimization and feature enhancements could further improve its utility in aiding healthcare professionals with prompt diagnosis and treatment decisions.