Abstract:
The importance of MRI image segmentation is rapidly growing in medical imaging. It facilitates tasks like identifying anatomic structures of organs, as well as detecting tumors and illnesses in early stages, facilitating the analysis of anatomy and pathology.
For a long time, clinicians have relied strongly on manual segmentation. Though important, manual segmentation of cardiac MRI images is generally very time-consuming, labor-intensive, and prone to inter-observer variability; thus, this creates a strong need for automated solutions.
In this project, we tried to work on and improve an MRI image segmentation network by using the infamous ACDC dataset, which has become a standard for benchmarking automated cardiac segmentation methods. It provides multi-class ground truth annotations of the left ventricle, right ventricle, and myocardium, along with diverse MRI images that capture variations in both anatomy and pathology.
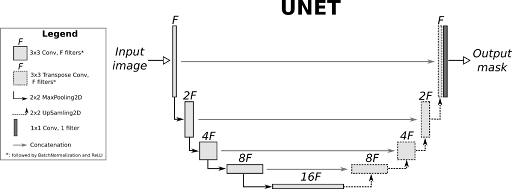
- The architecture selected for this review is the U-Net, a CNN that is widely used in biomedical image segmentation tasks. Some important features of the architecture are:
Encoder: A set of convolutional blocks with max-pooling operations to extract hierarchical features at different resolutions.
Decoder: Upsampling operations combined with skip connections to recover spatial details lost during encoding.
Skip Connections: Allow spatial information to be transferred directly from encoder layers to decoder layers for better segmentation quality.
Output Layer: A convolutional layer that maps the features to the number of segmentation classes, producing a probabilistic mask for each class.
The U-Net implementation has the following details:
Input Channels: Single-channel grayscale MRI images.
Output Channels: Four classes representing the background, left ventricle, right ventricle, and myocardium.
Layer Details: Convolutional blocks with ReLU activations and Batch Normalization.
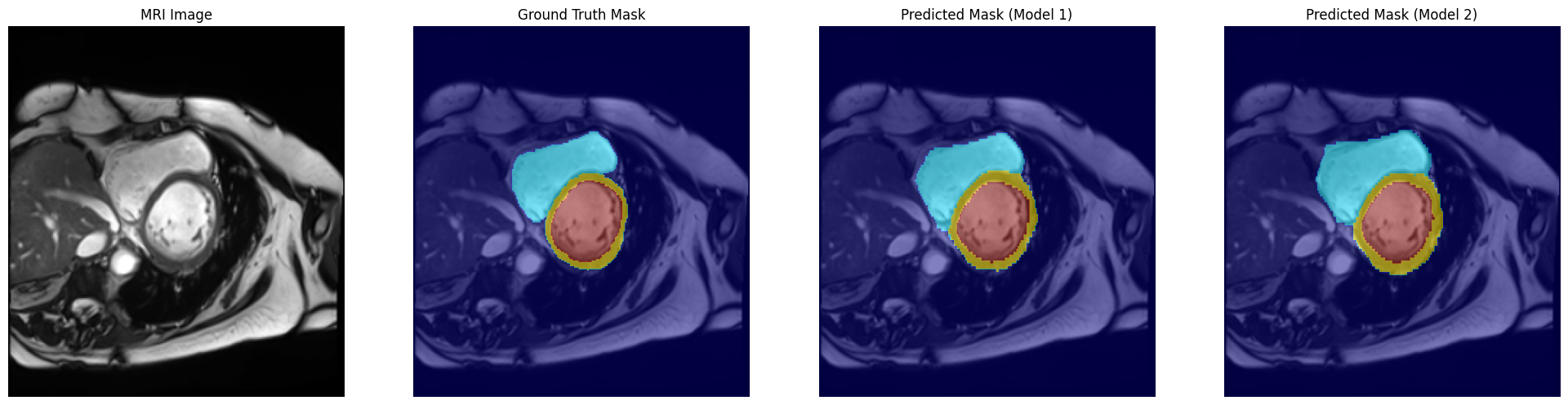
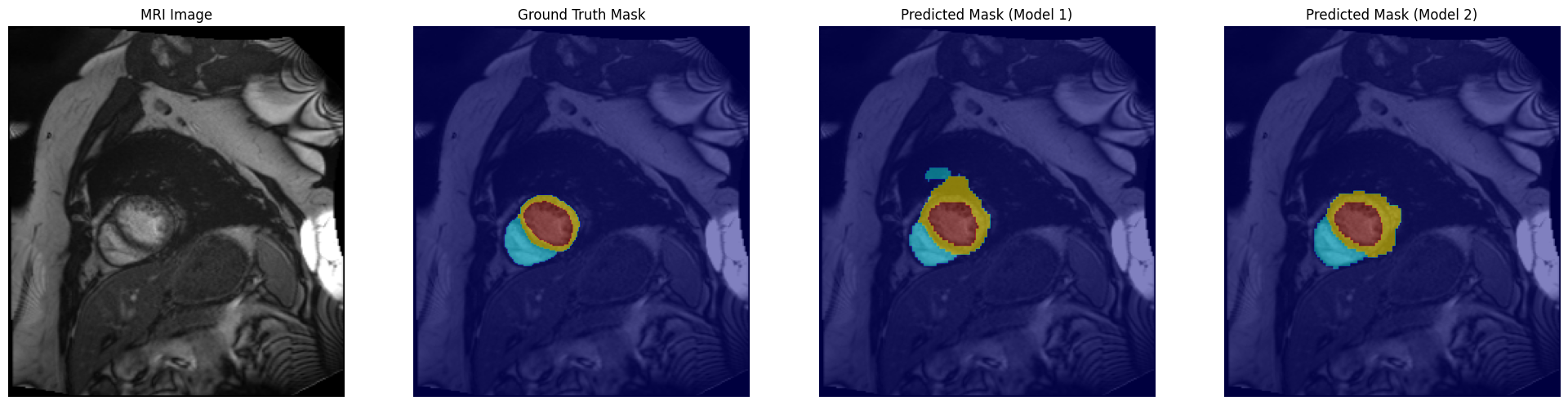
Evaluation and Results:
We assume that:
Model 1: un-weighted cross entropy loss, no data transformation is applied on the images and their masks.
Model 2: weighted cross entropy loss, data transformation is applied on the images and their masks.
Evaluation of the segmentation models was performed both quantitatively and qualitatively. All models were tested on a held-out test set of the ACDC MRI dataset to measure their performance in segmenting the cardiac structures. The key metrics that will be used for evaluation include pixel-wise accuracy and Dice scores for each class. Besides, visual comparisons of predicted masks with the ground truth were employed to analyze strengths and weaknesses of the models.
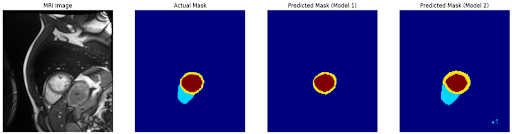
Combined Visualisation:


Conclusion:
In this study, we explored the task of cardiac MRI segmentation using a U-Net architecture on the ACDC dataset. Starting with a baseline "vanilla" model, which used standard cross-entropy loss and no data augmentation, we systematically introduced enhancements such as weighted cross-entropy loss to address class imbalances and data augmentations to improve model generalization. These refinements aimed to tackle the inherent challenges in medical image segmentation, including handling small structures of interest (e.g., myocardium and ventricles) and avoiding over-segmentation or false positives.
Key Findings
Baseline Model Performance: Baseline performance was pretty decent with overall high accuracy due to the dominance of background pixels, while Dice scores showed that the smaller structures such as myocardium and right ventricle are undersegmented, thus showing the struggles of the model in class imbalance and variability of data.
Improvement with Weighted Loss: Adding class weights to the cross-entropy loss function did allow for improved capturing of the smaller classes, as reflected in the marginally higher Dice scores observed for these regions. This also resulted in more over-segmentation, in the form of a greater number of false positives, particularly in background regions.
Impact of Data Augmentation: Data augmentation like random rotation, flipping, and brightness adjustment increased the sensitivity of the model to changes in the input data, hence giving better coverage of the target regions. However, this sometimes introduced noise in the model, resulting in inconsistent performance on edge cases.
Strengths and Limitations
The improved model demonstrated better sensitivity and generalization than the baseline model, making it more robust for clinical use. However, the improvements in Dice scores were modest, and the model remained prone to over-segmentation in certain cases.
Qualitative visualizations showed that while the models captured the general shape of cardiac structures, they often struggled with fine boundary details, suggesting room for further refinement in the architecture or training process.
Future Directions
Advanced Data Augmentation: Incorporating more sophisticated augmentations, such as elastic deformations or domain-specific transformations, could provide additional benefits in handling data variability.
Loss Function Refinements: Exploring alternative loss functions, such as a combined Dice and cross-entropy loss, could help balance overlap accuracy with boundary precision.
References:
ChatGPT. (2024). Used for code generation, comment generation, text generation, and text correction throughout this project. OpenAI. Available at: https://openai.com/chatgpt.
An Exploration of 2D and 3D Deep Learning Techniques for Cardiac MR Image Segmentation
Semi-Supervised Learning With Fact-Forcing for Medical Image Segmentation
A deep learning-based approach for automatic segmentation and quantification of the left ventricle from cardiac cine MR images
Transformer and group parallel axial attention co-encoder for medical image segmentation
GPA-TUNet: Transformer and GPA Attention Co-Encoder for Medical Image Segmentation